Research
PTSD
Fear is an essential emotion for survival. However, when fear is sustained and disproportionate to the extent of the threat, the maladaptive response can lead to fear disorders, e.g. post-traumatic stress disorder (PTSD).
Patients with fear disorders show a shift in excitatory to inhibitory (E/I) balance in the brain. Neurotransmitter switching, known as the gain of one neurotransmitter and loss of another in the same neuron, is a form of plasticity that regulates E/I balance and may control sustained fear. We have discovered a subset of serotonergic neurons in the lateral wings of the dorsal raphe of adult mice that switch their co-transmitter from glutamate/vGluT3 to GABA/GAD67 after intense footshock stress. Shocked mice show fear-related passive behaviors as they reduce exploration and increase freezing in a novel environment. We’re investigating whether the loss of glutamate/vGluT3 and gain of GABAGAD67 occurs in the same neurons and studying the causal relationship between the transmitter switch and sustained fear. We’re also testing whether fluoxetine, a selective serotonin reuptake inhibitor (SSRI) that is a first-line pharmaceutical treatment for fear disorders, affects transmitter switching and suppresses sustained and inappropriate fear. We expect to identify brain plasticity that links a prior strong fearful experience to subsequent long-lasting fear and passive behavior, and provide mechanistic understanding of the action of a pharmacological treatment for fear disorders.
Autism

Neurodevelopmental disorders including autism spectrum disorders are characterized by varying degrees of enhanced stereotypic behaviors, deficits in social interaction, and defects in communication. Early environmental factors play a key role and cortical neurotransmitter imbalance is a convergent phenotype seen across different types of these disorders.
Neurotransmitter switching involves loss of one transmitter and gain of another. It often changes the sign of the synapse from excitatory to inhibitory or vice versa and causes changes in behavior. We are testing the hypothesis that transmitter switching contributes to developmental disorders in mouse models. We treat pregnant females with either Poly Inosine:Cytosine (Poly I:C) or valproic acid (VPA) and study the development of the pups. We find a transient decrease in GAD67+ neurons and an equal increase in vGluT1+ neurons in the medial prefrontal cortex (mPFC) perinatally in experimental mice compared to controls. The switch is transient and reverses; it has disappeared in adults, although they demonstrate enhanced stereotypy and deficits in social interaction compared to controls. We then generated a mouse line to virally label neurons with GFP and demonstrate transmitter switching in single neurons in response to maternal Poly I:C treatment in both male and female mice, providing strong evidence for transmitter switching. To test the role of the transmitter switch in emergence of behavioral disorders, we restored GAD67 specifically in neurons that had lost it, using viral vectors. Our previous work showed that overriding one of the two transmitter changes in a switch is sufficient to prevent the change in behavior. Restoring GAD67 in the mPFC rescued altered behavior in experimental male mice. Our results add to growing evidence that alteration in signaling in the nervous system during the early stages of its construction can be detrimental to the function of the mature brain.
Drugs of Abuse

Continual exposure to drugs of abuse induces multiple forms of brain plasticity that play a role in the appearance of maladaptive behavioral alterations. However, the full repertoire of plastic changes involved and the mechanisms through which they influence behavior are still unknown.
Neurotransmitter switching is an environmentally-induced form of plasticity not previously studied in association with drug abuse, in which activity causes a subset of neurons to stop expressing the transmitter they were expressing before and/or express a different one. Our study aims to understand if neurotransmitter switching can be caused by drug exposure and contribute to drug-induced behaviors. We used mice expressing a Cre recombinase under the promoter of vGluT1 and a Cre-dependent mCherry reporter to permanently label glutamatergic neurons. Repeated administration of the addictive drug phencyclidine induces a subset of glutamatergic mCherry+ excitatory neurons in the prelimbic cortex of adult mice to switch their neurotransmitter from glutamate to GABA. They gain the inhibitory transmitter GABA and its synthetic enzyme GAD67, as demonstrated immunohistochemically, and decrease the expression level of vGluT1, as shown by RNAscope. Remarkably, when we override the ability of glutamatergic neurons to gain GABA by injecting a Cre-dependent AAV expressing GAD1shRNA in the prelimbic cortex of these mice prior to exposure to phencyclidine, we observe a rescue of cognitive deficits in the novel object recognition test, the spontaneous alternation task, and the loss of drug-induced sensitization to the locomotor effects of the drug. This evidence establishes a causal link between the gain of GABA in glutamatergic pyramidal neurons and phencyclidine-induced behavioral alterations. We are now investigating less invasive strategies to prevent the drug-induced gain of GABA and rescue the behavioral deficits.
Normal Brain

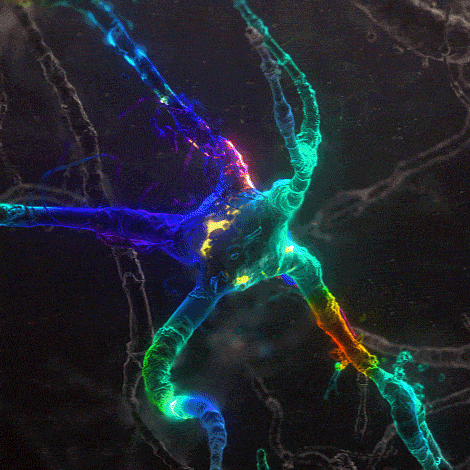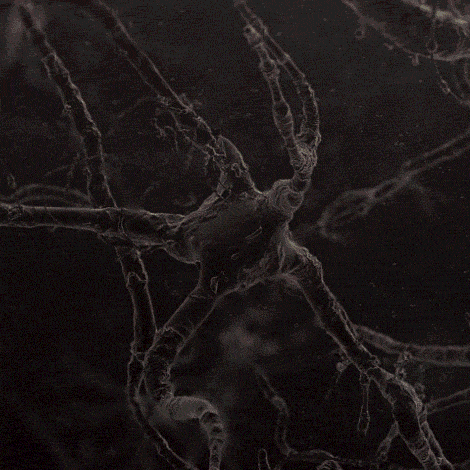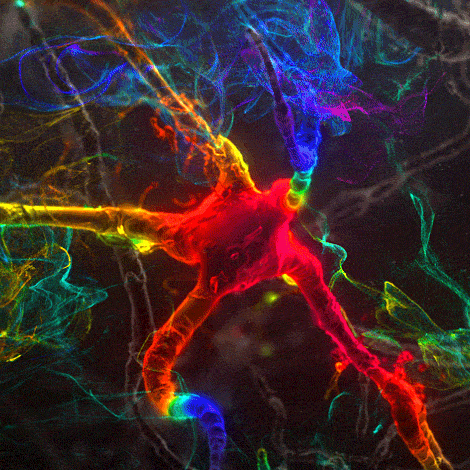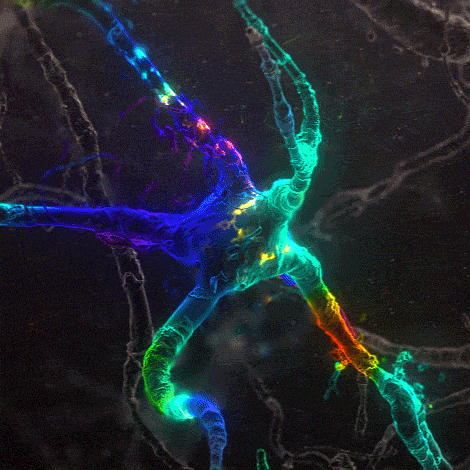
Many environmental stimuli contribute to beneficial neuroplasticity but the molecular and cellular changes in the brain remain obscure in most cases.
Motor control is required for accurate movements and can be disrupted in natural aging or motor disorders such as ataxia. Exercise is a therapy that improves motor skill learning. We found that adult mice given free access to running wheels for a week acquire enhanced motor coordination compared to non-runner controls, exemplified by a higher speed at fall from an accelerating rotarod and a shorter time to cross a balance beam. This was associated with the disappearance of a population of ChAT+ neurons and the appearance of an equal number of GAD1+ neurons in the caudal pedunculopontine nucleus of runners compared to controls. No neuronal death or neurogenesis was detected. We used a ChAT-Cre mouse line to label ChAT+ neurons with Cre-dependent viral expression of fluorescent mRuby2 and discovered that single ChAT+ neurons are losing ChAT and gaining GABA expression. To test for causality between the transmitter switch and behavioral change, we overexpressed ChAT or suppressed expression of GAD1 in the cPPN by injection of ChAT-Cre mice with Cre-dependent viral constructs. We found that both of these transmitter-rescue experiments prevented the improvements in motor coordination gained by running. These results suggest that transmitter switching is necessary for the enhancement of motor skill learning.
One week of running also induces disappearance of neuropeptide Y (NPY)-expressing neurons and appearance of an equal number of vGluT1-expressing neurons in the hilus of the dentate gyrus of adult mice. The loss of NPY neurons was associated with a decrease in the NPY fiber density in the molecular layer of the dentate gyrus. No cell death or neurogenesis was associated with these changes in the hilus although increased neurogenesis was observed in the subgranular zone as expected. Running increases episodic memory, evaluated with object recognition and contextual fear conditioning tests, even after animals have been resting for one or two weeks, suggesting that NPY-Glu switching contributes to a long-lasting effect on circuits in the dentate gyrus. We are now using an NPY-Cre mouse line to determine whether the transmitter switch occurs in single neurons. To dissect the involvement of the switch in the regulation of neurogenesis and the increase memory performance we will prevent the loss of NPY and the gain of glutamate expression using Cre-dependent viruses expressing NPY or a small interference vGlut1 stereotaxically injected into the dentate of NPY-Cre mice before the onset of the running period. We hypothesize that this transmitter switch is necessary for the improvement in episodic memory